Since the 1990s, dozens of rational drug design packages have been published. These programs fall in one of three main genres: 1. Scanners, 2. Builders, or 3. Hybrids.
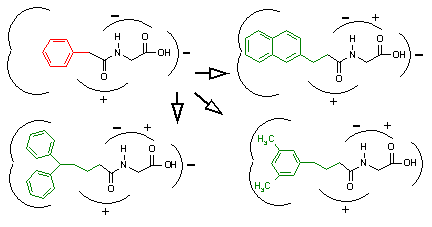
Although builder-type programs may be used for denovo ligand design, their chief utility is in the optimization of lead compounds. These programs also utilize a database of structures; however, the database contains fragments and chemical building blocks instead of complete compounds. In order to use a builder-type program, the user must possess a lead compound that requires optimization. Such compounds normally contain an area that poorly complements the corresponding receptor region. This is illustrated in the upper left corner of Figure 9. Here we see a lead compound that contains a stable, tight-binding region (black), and a phenyl ring that should be replaced to improve receptor complementarity (red). Builder-type programs require the attachment point of the weak-binding portion as input. The software then removes the offending ligand region and uses the attachment point to create a population of derivatives by adding, deleting, and substituting fragments chosen from the component database (green) to fill the active site. The binding energies of the resulting derivative ligands are then calculated. Those structures that augment binding are retained while those that do not are discarded. This process repeats as the new population of structures is then processed to generate the next round of derivatives. By making incremental changes iteratively, these programs generate a set of ligands with improved receptor complementarity over time.

Figure 9. Demonstration of
Builder-type programs.
There are several advantages in using these types of programs. First, no database of structures is required, allowing anyone to use these programs without additional requirements. Although a component database is utilized, this is often built into the software. The combinatorial addition of fragments offers a vast number of potential derivative structures. Because components from numerous chemical classes are included, these programs automatically generate a diverse set of chemical solutions. This also contributes to the creation of truly novel ligands. Finally, these programs lend themselves well to the current drug development pipeline, since they can be utilized to optimize the hits that result from mass screening.
However, the strengths described above for builder-type programs lend to their weaknesses. With the combinatorial attachment of such diverse chemical components, the generation of synthetically unfeasible structures is commonplace. Furthermore, ligands that are chemically unstable are produced as well. Although a diverse set of chemical building blocks are utilized, the manner in which they are attached is entirely up to the developer of the software. Decisions such as when a particular component is selected and where it is attached greatly affect the generated structures. These choices reflect the bias of the program developers. In addition, the success of any builder program to generate improved ligands is entirely dependent upon its ability to accurately calculate the receptor-binding structure and binding affinity. As we have stated above, this is a very challenging problem.
Prev - Scanners
Next - Conformational Searching
Return to RACHEL Technology - Main
(c) 2002 Drug Design Methodologies, LLC. All Rights Reserved