Given our introduction to the biochemistry of ligand receptor binding, we can begin to appreciate the difficulties in designing drugs towards specific target receptors. Table 1 lists the major tasks and concerns in this endeavor.
|
Table 1. Major tasks and concerns in drug development. |
|
1. Characterize medical condition and determine receptor targets. |
|
2. Achieve active site complementarity: steric, electrostatic, and hydrophobic. |
|
3. Consider biochemical mechanism of receptor. |
|
4. Adhere to laws of chemistry. |
|
5. Synthetic feasibility. |
|
6. Biological considerations. |
|
7. Patent considerations. |
When a medical condition exists where a drug could be beneficial, extensive scientific study must first be done in order to determine the biological and biochemical problems that underlie the disease process. This often takes years of study in order to characterize the targets for a potential drug. The reason is that nearly all biological processes in the human body are tightly interconnected. Altering the behavior of select receptors or enzymes may have detrimental effects with other systems. These are the side effects that occur with nearly all drugs. Furthermore, the human body is a homeostatic machine, and always attempts to achieve equilibrium. As a result, the body will attempt to counteract any pharmacotherapeutic intervention.
Once a receptor target has been established
and well characterized, the process of ligand design begins. Obviously,
the first consideration is that the designed ligand must complement the active
site of the receptor target. Steric, electrostatic, and hydrophobic
complementarity must be established as we discussed above. The
pharmacophore must be presented to the receptor in order for recognition and
binding to occur. Otherwise, the designed ligand will have no chance of
interacting with the receptor.

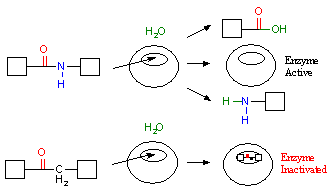
Figure 4. Designing ligands to offset enzyme mechanism.
In addition to adequately binding the receptor, the biochemical mechanism of the receptor target must be taken into consideration. This is shown in Figure 4. In this figure we schematically represent the biochemical mechanism of a protease. A protease is an enzyme that cleaves proteins and peptides. In the top part of the figure, we see that a protease recognizes a specific group of atoms, colored in red and blue, called a peptide bond. If the peptide bond is present at a specific position in the active site when the ligand binds, it is cleaved by the protease with the addition of water (H2O) to form two separate fragments. If our goal is to inactivate this protease, any designed ligand cannot possess this peptide bond at the same position. Otherwise, it will simply be cleaved by the protease, and the protease will continue to function unperturbed. However, the ligand can be modified so that the peptide bond is no longer present as shown in the bottom portion of the figure. If this ligand is then bound by the enzyme, the enzyme will not be able to cleave it. As such, the enzyme would be inactivated, as the ligand remains lodged in the active site.
Having characterized the active site region and the mechanism of action of the target receptor, the challenge then becomes one of designing a suitable ligand. This is, by far, the most daunting task of the entire drug design process. The optimal combination of atoms and functional groups to complement the receptor is often the natural ligand of the receptor. Unfortunately, this is usually an unacceptable candidate for a drug. This is because the natural ligand either fails to inactivate the receptor, as described above, or it is a natural substance that cannot be patented. Patent considerations are often paramount, as legal protection for the developed drug affords the opportunity to recoup the financial costs of development. Therefore, alternate combinations of chemical structures must be devised.
The design of novel ligands is often restricted by what chemists are physically able to synthesize. It is of no use to design the ultimate drug if it cannot be manufactured. The laws of chemistry dictate that each atom type has a specific size, charge, and geometry with respect to the number and types of neighboring atoms that it can be joined to. The entire field of chemistry is predicated on the establishment of synthetic rules for the construction and manipulation of various combinations of atoms and functional groups. It is the expertise in these chemical rules that govern the ability of the synthetic chemist to design and synthesize postulated ligand candidates. Within these rules, the drug developer must creatively propose suitable chemical structures that satisfy the requirements discussed above.
Finally, there are biological considerations to the development of new drugs. The liver is the major organ of detoxification in the human body. Any drug that is taken undergoes a number of chemical reactions in the liver as the body attempts to neutralize foreign substances. This set of reactions is well characterized, and a great deal of knowledge exists as to how drugs are modified as the body eliminates them. More importantly, various chemical structures are highly toxic to biological systems, and these are also well characterized. These constraints must also be taken under consideration as novel drugs are developed.
Prev - The Biochemistry of
Drugs
Next - The Drug Discovery Pipeline
Return to RACHEL Technology - Main
(c) 2002 Drug Design Methodologies, LLC. All Rights Reserved