The ability to instantly cross-reference components by chemical composition also permits user-directed structure generation, which is the most powerful and unique feature of RACHEL. No other builder-type drug design package incorporates this feature. Essentially, this technology permits the true application of virtual combinatorial chemistry. The inspiration for this technology stems from earlier work published by the author - 'DBMAKER: A set of programs to generate three-dimensional databases based upon user-specified criteria' Ho, CMW., and Marshall, GR., J. Comp-Aided Mol. Design, 9:65-86 (1995).
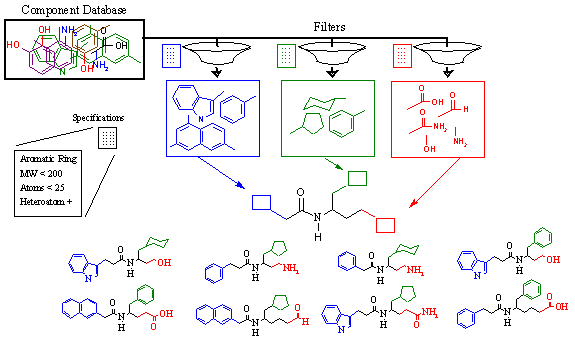
Figure 14A demonstrates this with an example. In the middle of this figure, we see a lead compound scaffold containing an amide bond with various side chains extending from it.

Figure 14A. RACHEL user-directed structure generation.
From biochemical characterization of this lead compound we discover that three chemical groups make up the pharmacophore. The first group, shown in blue, must contain a large ring system. Crystallographic analysis reveals that both single and bi-cyclic rings are capable of binding, as long as they are planar. Thus, they must be aromatic. Any atom types may be accepted. The second group, shown in green, has different requirements. Again, a cyclic component is desired. However, the binding pocket in this region is smaller, but more spherical. Thus, only single rings are acceptable although they need not be aromatic. In addition, this region is very hydrophobic; thus, only hydrocarbon components are acceptable (only carbon and hydrogen). The third group, shown in red, is quite different from the first two. This region of the active site is highly charged, and requires a small polar group to interact with. Thus, no ring structures are acceptable. Furthermore, heteroatoms (nitrogen, oxygen) are required.
|
BLUE derivatives |
GREEN Derivatives |
RED Derivatives |
|
(+) Ring structures - aromatic |
(+) Ring structure - single |
(-) Ring structures |
|
Molecular weight < 200 |
Molecular weight < 100 |
Molecular weight < 50 |
|
# Atoms < 25 |
# Atoms < 20 |
# Atoms < 8 |
|
(+) Any atom type |
(+) C, H = only |
(+) N, O = required |
Table 5. Chemical requirements for each
derivative group in Fig 14A.
The various chemical requirements of each derivative group are summarized in Table 5. In order to implement these requirements, a component specification language has been developed. This specification language contains a combination of keywords, target values, and Boolean operators. A brief summary of these commands is listed in Table 6 below. The specification language is very powerful and allows the user to control these characteristics and many more. Once the chemical requirements are established for each derivative group, RACHEL then filters the master component database and generates individual databases for each subsite.
|
Command |
Function |
|
CMPNTS min - max |
Number of total components to utilize. |
|
ATOMS min - max |
Number of atoms in a specific component. |
|
R-ATOMS min - max |
Number of ring atoms in a specific component. |
|
MW min - max |
Molecular weight. |
|
LINKS atypes (<,>,=) value |
Specifies rotatable bond atom types between components. |
|
ATYPES (list) (<,>,=) value |
Specifies atom type requirements in a specific component. |
|
BONDS (list) (<,>,=) value |
Specifies bonded atom types within a specific component. |
|
PHARM (atype) {x,y,z} |
Specifies a specific pharmacophoric group at coordinates {xyz}. |
Table 6. RACHEL component specification language.
Using these individual databases, shown in Figure 14A as the blue, green, and red boxes, RACHEL combinatorially generates all possible derivatives within the constraints of the active site. In so doing, an immense number of diverse chemical structures may be constructed and tested in a defined and controlled manner.
As we have demonstrated, the user has considerable control over the chemical species that can be incorporated into structures. However, another feature unique to RACHEL that the component specification language permits is the removal of undesired structures. As stated above, this is one of the shortcomings in computer-aided drug design, especially with builder-type programs. Builders can often generate combinations of components that are neither stable nor synthetically feasible. The terms listed in Table 6 may be used in a variety of ways to limit the generation of these unacceptable structures. This is illustrated in Figure 14B below.
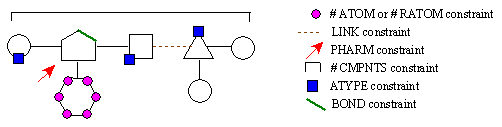
Figure 14B. RACHEL component constraints.
We see here the various constraints RACHEL can employ and how they relate to a given structure. In this hypothetical chemical structure, the geometric shapes represent different components while the lines connecting them correspond to rotatable bonds. The ATOM and RATOM constraints govern how many atoms a particular component can possess. The LINK constraint limits the atom types that can be utilized in rotatable bonds. The PHARM specification signifies that a specific atom type must be present at a precise location in the active site. The #CMPNTS restriction places upper and lower bounds on the total number of components a structure can possess. The ATYPE constraint stipulates how many atoms of a specific type can be present in both individual components as well as the entire structure. The BOND specification places limits on the bonded atom types that can be present within a component. As one can see, this again gives the user a tremendous amount of control over the structures generated by RACHEL.
An additional feature that is unique to RACHEL is an automated method to generate diversity using templates. These templates are an integral part of the component specification language. As is normally the case, a computational chemist is not sure exactly what derivative components might complement the receptor. However, specific chemical groups may be desired at general locations in the active site. These might be pharmacophoric elements, or proprietary chemical structures for which synthetic methods are available. This is illustrated in Figure 14C below.
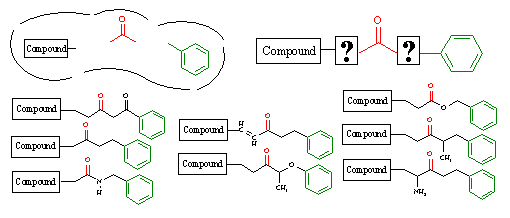
Figure 14C. Template driven structure generation.
In the upper left, we show an active site that contains a portion of a lead compound that has been previously characterized. We also know that two chemical groups, a carbonyl group (red) and a phenyl ring (green), are required to satisfy the pharmacophore for ligand binding. Given these issues, RACHEL allows the user to define a chemical template, as shown in the upper right of this figure, to generate appropriate structures. In this template the lead compound fragment and the two pharmacophoric groups are separated by wildcard designations, which denote where chemical variability can occur.
RACHEL will then generate chemically diverse structures using the template as shown in the figure above. The static portions of the template are left untouched, and they are incorporated into every generated derivative. However, the wildcard regions allow RACHEL to creatively insert various components in a random manner to link these pharmacophoric elements together. Obviously, constraints can be placed on these variable regions using the component specifications described above. As such, the use of these templates enables RACHEL to fully explore the chemical diversity within the corporate database and maintain the fundamental groups necessary to achieve receptor binding.
Prev - Intelligent Component Selection
Next - Scoring Functions
Return to RACHEL Technology - Main
(c) 2002 Drug Design Methodologies, LLC. All Rights Reserved